GlobalVigiPub provides you the free online pharmacovigilance training via the posts page on our website. This Guidance on drug safety is good enough to understand the Pharmacovigilance basics and get the roots of pharmacovigilance in detailed too. Just browse the posts and read to get the knowledge on Pharmacovigilance course
General Pharmacovigilance Guidelines in Switzerland
Q: What is update on reporting system of adverse drug reactions by healthcare professionals?
A: From 1 January 2021, healthcare professionals should report adverse drug reactions directly to Swissmedic.
Q: What is update on electronic reports of adverse drug reactions?
A: From 01 July 2021, only electronic reports of adverse drug reactions will be accepted from marketing authorisation holders either via a gateway connection or via the ElViS portal (Electronic Vigilance System)
Readers Note: if you really wants to rid of Alcohol, Just 7 Days to Drink less. Join in Online Alcohol Reduction Program, Alcohol Free Forever
Q: How pharmaceutical companies (MAH) does reporting/submitting suspected adverse drug reactions in Switzerland?
A: Marketing Authorisation Holders (MAH) basically have two options for submitting suspected adverse drug reactions to Swissmedic.
- Electronic reports via the ElViS portal (Electronic Vigilance System) Submitting reports via a web-based online tool
- Gateway for the electronic exchange of individual case safety reports. The reports are transferred directly to Swissmedic’s database
Registration processes are required for both options
Readers Note: Intensive Stretch Mark Therapy with Skinception is noted treatment for years to come
Q: What is registration via ElViS and how to do?
A: Submission via ElViS is intended for use by small to medium-sized pharmaceutical companies without their own access (via Gateway) to Swissmedic’s pharmacovigilance database.
Registration for MAHs as below
- The MAH registers for ElViS on the Swissmedic website:
- The Informatics Service Center of Swissmedic (ISCS) creates a contract
- The MAH signs the contract and delegates a Partner-Administrator
- ISCS creates the Partner-Administrator Account
Readers Note: Fight Back against Viruses and Inflammation and Promote Proper Immune System Function with Extract of Curcumin 2000
Q: What is registration via PV Gateway of Swiss and how to do?
A: Companies that are interested in using the Gateway solution and that fulfil the above requirements should send a letter of intent to pvgateway@swissmedic.ch After examining the letter, Swissmedic will contact the company to its involvement in detail.
Q: What are sources of Adverse event reporting in Switzerland?
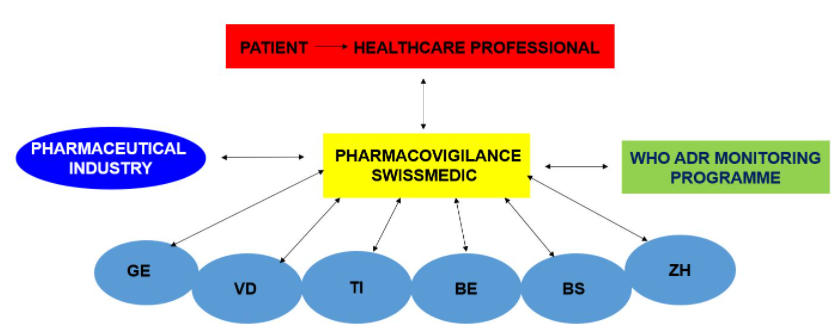
A: The Swissmedic National Pharmacovigilance Centre accepts and processes reports of adverse drug reactions from professionals. The National Pharmacovigilance Centre is supported by six regional centres. These centres process reports from healthcare professionals that involve an important safety signal.
Readers Note: A product is considered as standout product in the hair regrowth industry. Profollica can regenerate the follicular growth which results your hair back
Readers Note: Provillus Hair Loss Treatment. Why more people are turning to it to prevent hair loss for MEN, for WOMEN and regrow hair with the only FDA approved ingredient on the market
Flow chart as below
Readers Note: Natural support for your cholesterol. The Hypercet cholesterol Formula acts as a general tonic supporting the cardiovascular system. Supports good and bad cholesterol levels within the normal range along with the ability to deal with harmful free radicals
Q: When Swissmedic’s Pharmacovigilance Gateway for the exchange of individual case safety reports on adverse drug reactions went live?
A: In December 2012 Swissmedic’s Pharmacovigilance Gateway for the exchange of individual case safety reports on adverse drug reactions went live
Readers Note: If you would like to Quit Smoking? Really it’s a Magic. Get it with Stop Smoking Program, Smoke-free just in short span
Q: What is time line for reporting of serious and non-serious reactions?
A: These suspected adverse reactions should be reported within 15 days of diagnosis; non-serious reactions should be reported within 60 days.
Q: Who can report the adverse events and How to report those?
A: the below are the ways to report the Adverse events.
- Patients: Patient can find information on the reporting/submission of suspected adverse drug reactions by patients here.
- Healthcare professionals: Adverse drug reactions should preferably be reported electronically via the online ElViS (Electronic Vigilance System) portal.
- Pharmaceutical companies: You can find information on the reporting/submission of suspected adverse drug reactions by pharmaceutical companies here
Readers Note: The clinically proven and natural premium fitness supplements as PrimeGENIX for men over 40 who want to get in shape and have more energy
Q: What happens when a report is filed?
A: Reports from healthcare professionals are evaluated in the Swissmedic National Pharmacovigilance Centre and, in some cases, in the regional centres.
The primary reporter receives a confirmation of receipt of the report and a written evaluation if the report was forwarded to a regional centre for processing. All evaluated reports are completely anonymised and entered in the national database.
If reports are incomplete, it might be necessary to get back to the primary reporter via the regional centre. The pharmaceutical industry forwards any reports it receives directly to Swissmedic. Swissmedic, in turn, forwards all reports to the international centre for drug safety at the World Health Organization (WHO).
Readers Note: Try this unique eyelash growth serum Idol Lash that will help you achieve Longer, Darker, Thicker and Beautiful eyelashes in a matter of weeks
Q: Why can I only store one unfinalised report in ElViS?
A: ElViS is primarily a reporting platform, not a database. That’s why submitted reports are only stored in ElViS for a limited period of time. However, you can resume work on any unfinalised report that you store in ElViS whenever you want.
Q: How long will reports that I submit be stored in ElViS?
A: No more than 6 weeks. It is the responsibility of each user to archive their reports and accompanying documents on their own computer.
Readers Note: A specially designed weight loss programs is combined with an exciting new breakthrough ingredient that comes with a complete online comprehensive diet and weight loss programs to help you lose weight. Here are Garcinia UltraPure, New Tonic Supplement, Viva Slim liquid drops, 30 Days to weight loss program to burn fat while eating foods you love using home workouts
Q: Are reports submitted via ElViS also sent to the Regional Pharmacovigilance Centres?
A: Yes. Any reports you submit via ElViS will be sent to one of the Regional Pharmacovigilance Centres.
Q: Whom do MAH contact if they have questions or problems regarding ElViS Account?
A: Please contact the ElViS hotline by e-mailing it@swissmedic.ch or by calling +41 58 462 06 00. The hotline is open from 7.30 a.m. to 5.30 p.m., Monday to Friday.
Q: Why do MAH have to register?
A: Registration is required before MAH can use ElViS. This is to ensure that the data you submit are transmitted securely and to unequivocally link senders to reports. Registration doesn’t take long and all the information you provide will be treated in strict confidence.
Readers Note: A complete protein diet. Pure liquid egg whites are the purest form of protein known to man in the entire world and are 100% bio-available
Q: EIViS Account registration by MAH?
A: MAHs should register for ElViS on the Swissmedic website. A ticket will automatically be created at Swissmedic and the request to use ElViS will be processed. Once Authority have reviewed the information provided, MAH will receive an ElViS user agreement.
Swissmedic registration portal as Registration Swissmedic Portal
Q: What’s the difference between a “direct insert” and a “file upload”?
A: If you want to type your report into an online entry mask, choose “direct insert”. This is the standard option for most users. If you have an electronic system capable of generating E2B files and you want to upload these files to ElViS, choose “file upload”. ElViS can only be used with the E2B-R2 format. This option will normally only be available in specialised centres. You will have to contact the ElViS hotline before you can start a file upload. The same applies if you have chosen a file but then want to change it.
Readers Note: A unique 2-step process releases free oxygen to oxidize and lift organic stains away from teeth. Let us begin this patented dental whitening system, Teeth Whitening 4 You to remove stains with only one use
Readers Note: Find a revolutionary scientific breakthrough for controlling unsightly cellulite and eliminating inches with Dermology Cellulite Cream
Readers Note: Do you find any of natural herbal colon cleansing treatment that has changed hundreds of thousands of people’s lives? Yes, here is a through and effective internal cleansing with Bowtrol

Itís difficult to find educated people about this topic, but you seem like you know what youíre talking about! Thanks